Fighting to endTB, One Patient at a Time
29th November 2019
Disclaimer: Names and identifying details have been changed to protect the privacy of individuals.
The global trend points to a growing burden of multidrug-resistant tuberculosis (MDR-TB). In 2018, there were about half a million new cases of rifampicin-resistant TB (of which 78% had MDR-TB). Alarmingly, only 38% of these cases were detected and notified.1
But these cases are more than just statistics – they are human beings, each with their own individual experiences and stories.
MDR-TB drugs affect every patient differently. In some cases, patients may go through months of treatment, only for a regimen not to work. Others go through numerous obstacles to find treatment, and if they do, many are unable to tolerate it because of its many harmful side effects, in addition to having to swallow a handful of pills daily (over 20 a day) consecutively for almost two years, along with daily injections.
A multi-country endTB clinical trial, newly launched in Pakistan, aims to address these issues and find more effective, shorter, less toxic, injection-free regimens for MDR-TB.
“The kind of treatment that is in this trial should be available everywhere.”
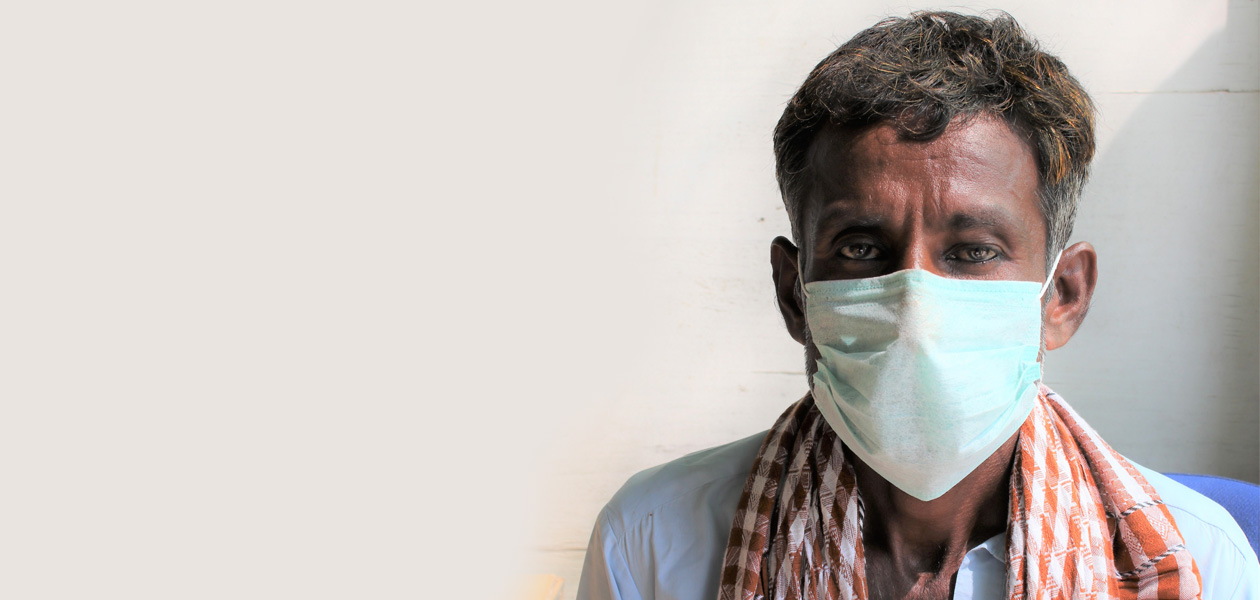
In October, we enrolled our very first patient — Asif. This is his story.
Asif resides in Thatta, Sindh. Once a hub of commerce and culture, Thatta has since been neglected by local and national authorities and the community has fallen to poverty and illiteracy.
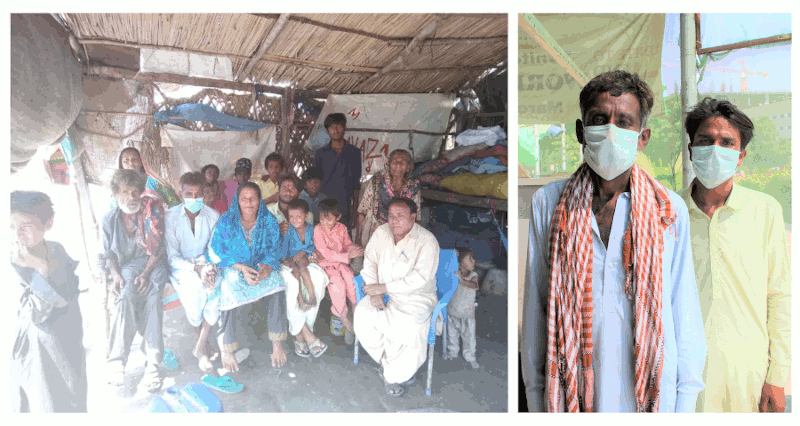
In this town of 220,000 people, Asif had been working at the same job for over 15 years, pushing a cart around collecting trash. But when he was diagnosed with MDR-TB about 4 months ago, he was forced to stop working and the burden of being the breadwinner shifted to his eldest, 18 year-old son Bilal who dropped out of school to support his father’s treatment and family.
Bilal earns about Rs. 300-400 a day (approximately $2-$3), and with this minimal income, they must support an entire family of 10, with another member on the way.
Living under slums, they make do with limited electricity and water supply, and cook over a wood fire. A car is a distant luxury, so they must rely on inefficient forms of public transportation. And though they barely make ends meet, the family is putting all their children through school.
This is just a glance of the hardships faced by impoverished communities. Because of barriers to accessing care, they lack formal education and job opportunities. They have limited knowledge of healthy lifestyle choices and little access to adequate healthcare — the closest hospital is 30 minutes away.
“I want to be free from this illness and be healthy again.”
So when Asif was diagnosed with MDR-TB, his immediate reaction was fear — whether or not he would find treatment, how he would pay for it and what the treatment would entail.
He was back and forth between hospitals looking for treatment, but none of the options were ideal. One hospital even told him he would have to be admitted for six months.
Finally, he was referred to The Indus Hospital, where he found out about the clinical trial. He immediately decided to participate — he wanted to get better no matter what. “I want to be free from this illness and be healthy again,” he said.
Asif remains positive and in high spirits, and is confident about the outcome of this trial. He is committed to seeing it through, because he understands many patients do not.
“We want him to get better, so that he can return to his life as it was before,” says his family.
Over the course of the next few months as part of the clinical trial, Asif will receive a treatment regimen for MDR-TB that is shorter than the usual treatment of almost 2 years. His regimen will not include painful injections, avoiding severe side effects such as deafness that accompany older MDR-TB treatments. He is also on nutritional support.
This trial is part of a multi-country effort by endTB – a partnership between Interactive Research and Development (IRD), Partners In Health (PIH), Médecins sans Frontières (MSF) and its financial partner UNITAID. The trial will enroll patients over the course of the next year and follow these patients through 2022. Through a series of posts, we will follow Asif’s journey and hope to tell the stories of other patients as they participate in this trial.
This piece was authored with the help of our Treatment Coordinator, Muhammad Yaseen.
References:
- WHO Global Tuberculosis Report 2019.
https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1

 Contact Us
Contact Us





 info@ird.global
info@ird.global
 16 Raffles Quay, #16-02, Hong Leong Building, Singapore 048581
16 Raffles Quay, #16-02, Hong Leong Building, Singapore 048581
 Designed by The Brand Crew
Designed by The Brand Crew